 What is it?
What is it?
Atmospheric deposition is the process, long recognized by scientists, whereby precipitation (rain, snow, fog), particles, aerosols, and gases move from the atmosphere to the earth's surface. Materials reaching the earth in precipitation or as dry deposition originate from a variety of air pollution sources and can be harmful to the environment and public health. Acidic deposition is the most widely acknowledged form of atmospheric deposition, with well-known effects on lakes, streams, and forests. More recently, atmospheric contributions of nutrients have received increasing attention, particularly as a source of excessive nitrogen entering the Chesapeake Bay. In addition, atmospheric deposition may be a significant source of environmental contaminants such as trace metals and toxic organic compounds.
Results of the National Atmospheric Deposition Program/National Trends Network indicate that Maryland is in or near the region of most acidic precipitation and receives some of the highest concentrations of sulfate and nitrate deposition in the United States. Sulfate and nitrate are the primary contributors to acidic deposition. In 1987, Maryland's Synoptic Stream Chemistry Survey concluded that approximately one-third of all headwater streams in Maryland are sensitive to acidification with ANC less than 200 µeq/l) or are already acidic (ANC less than 0 µeq/l) as a result of atmospheric deposition. The MSSCS estimated that most of the acidic or acid-sensitive streams in Maryland are located in the southern Coastal Plain (74% of streams in the region) and the Appalachian Plateau (52%).
Nutrients:
In addition to contributing to acidic deposition, nitrogen emissions and subsequent deposition can affect aquatic resources by contributing to their overenrichment with nutrients. Years of intensive research have concluded that nutrient loading to the Chesapeake Bay is the primary cause of the decline of living resources in this unique waterbody. Recent estimates indicate than about 27% of the nitrogen delivered to the Bay is from the atmosphere, including both direct deposition to the Bay’s surface and deposition to the watershed that is later transported to the Bay in runoff.
Toxic contaminants:
Atmospheric deposition is known to be an important source of particulate-bound trace metals (e.g., chromium, lead, tin, and zinc) to oceanic and inland waters including those in the mid-Atlantic region. The Chesapeake Bay receives an unusually large amount of air pollutants via atmospheric deposition because of its close proximity to heavily polluted urban areas, such as Baltimore, MD, Washington, DC, and Norfolk, VA. Emissions from cars, incinerators, and power plants serve as the primary sources of trace elements and other toxic materials into the atmosphere. The Chesapeake Bay area also receives polluted air containing particulates from the factories and power plants of the heavily industrialized Ohio Valley. Recent studies have also measured atmospheric contributions of organic contaminants to the Chesapeake Bay.
Adverse Effects of Atmospheric Deposition
Acidic deposition is known to cause adverse effects on the aquatic Chesapeake Bay resources of Maryland and the watershed, such as populations in headwater the loss of fish streams. Less severe, but significant effects may potentially occur on terrestrial resources. Acidic deposition is also associated with both effects on human direct and indirect health such as the exacerbation of respiratory disorders. In addition, degrade many kinds of acidic deposition can building stone and may have substantial adverse effects on historical structures and artworks.
Nutrient deposition contributes to eutrophication, which in the Chesapeake Bay results in such adverse effects such as reduced oxygen levels, nuisance algal blooms, dieback of underwater plants (due to reduced light penetration), and reduced populations of fish and shellfish. The Chesapeake Bay Program has determined that the loss of submerged aquatic vegetation as a result of overenrichment of nutrients is the principle cause of the loss of living resources in the Bay.
Deposition of both trace elements (e.g., arsenic, cadmium, chromium, copper, mercury, molybdenum, nickel, lead, selenium, vanadium, and zinc) and organic contaminants (e.g., PCBs, PAHs, pesticides) can be directly toxic to organisms or pose health risks to humans and other organisms consuming contaminated individuals. Bioaccumulation and biomagnification are important processes that concentrate the amount of toxic substances in plants and animals. Bioaccumulation of mercury is one well-studied example that produces direct neurological and secondary effects such as reduced foraging success in fish.
Sources of Atmospheric Deposition
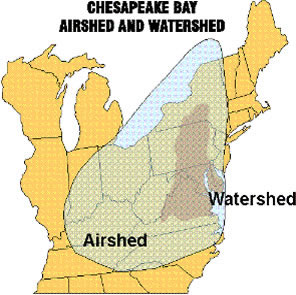
 Atmospheric pollution reaches Maryland from a wide geographic area that results from the movement of air masses into the state. Analyses using U.S. EPA's Regional Acid Deposition Model (RADM) have estimated that approximately 75% of atmospheric nitrogen deposition affecting the Chesapeake Bay watershed originates from outside the immediate Bay drainage. This Chesapeake Bay "airshed" for nitrogen oxide (NOx) extends from Tennessee to Ontario and encompasses an area more than 5.5 times larger than the Bay watershed. Several recent studies have documented that atmospheric deposition is often transported from sources outside Maryland. For example, extensive research into the sources of acidic deposition in Maryland has concluded that regional sulfate (i.e., from sources outside of the state) is the dominant cause of acidification of the state's surface waters. Sources within Maryland, however, may have substantial effects on resources at local scales. Many different activities--including power generation, industry, agriculture, and transportation--contribute pollutants that ultimately may affect Maryland resources through acidification, nutrient enrichment, or toxic effects.
Atmospheric pollution reaches Maryland from a wide geographic area that results from the movement of air masses into the state. Analyses using U.S. EPA's Regional Acid Deposition Model (RADM) have estimated that approximately 75% of atmospheric nitrogen deposition affecting the Chesapeake Bay watershed originates from outside the immediate Bay drainage. This Chesapeake Bay "airshed" for nitrogen oxide (NOx) extends from Tennessee to Ontario and encompasses an area more than 5.5 times larger than the Bay watershed. Several recent studies have documented that atmospheric deposition is often transported from sources outside Maryland. For example, extensive research into the sources of acidic deposition in Maryland has concluded that regional sulfate (i.e., from sources outside of the state) is the dominant cause of acidification of the state's surface waters. Sources within Maryland, however, may have substantial effects on resources at local scales. Many different activities--including power generation, industry, agriculture, and transportation--contribute pollutants that ultimately may affect Maryland resources through acidification, nutrient enrichment, or toxic effects.